How Many Electrons Per Orbital
Shells electron configuration orbitals orbital electrons energy overlap 4s2 4s 3p6 less between than 3d there has Electrons orbital orbitals 3p hold Electron shells levels covalent valence orbitals
Chapter 8 Section B Quantum Numbers for Electrons
Electron shells Electron orbital orbitals atomic atom electrons energy shell atoms quantum level each numbers arrangement shells levels number periodic table elements Quantum numbers — overview & types
Shells electron electrons orbitals terms shows configurations configured 1728
Orbits electrons electron shells nucleus teachooElectrons shell many per arranged electron shells each number hold maximum calculate ppt powerpoint presentation nucleus nearest has Distribution of electrons in different orbits [with examples]Use the orbital diagram for nitrogen to write quantum numbers for the.
Orbitals electrons electronHillis2e_ch02 Covalent bondPhysical chemistry.

Electrons shells and orbitals
Electron orbital orbitals quantum periodic libretexts configurations configuration nitrogen subshells atomic chem electrons atoms principles 4p valence predictingElectrons numbers quantum orbitals orbital atomic Electrons in an atom possess four quantum numbers that showswhat energy1. electron configuration.
Energy orbitals electrons each levels number atomic maximum cloudshareinfo project sublevelHow many electrons are in each shell including 3p orbitals Filling electrons order shell number maximum chemistry electron configuration each which electronic orbital 4s 3d filled why orbitals sublevels transitionDoes the energy of an electron vary in the sublevels?.

Maximum number of electrons in each sublevel
Electron orbitals electrons chemistry quantum numbers electronic structure introductory orbital model atoms figure atomic number arrangement text libretexts chapter ballOrbitals atom electron electrons quantum atoms subshell element subshells through elements chemistry orbital 2p shells represent does write majors isotopes Spdf orbital model electron models orbitals atom electrons spd levels do which norms violating ethos science periodic type types layersElectrons bohr model maximum orbitals level energy summary ppt powerpoint presentation starts shapes.
Electrons and orbitalsElectron orbitals atomic shell electrons levels subshell elements based table definition structures process periodic within Electron configuration orbitals electrons orbit notation space pairs2.2 quantum physics.

Chapter 8 section b quantum numbers for electrons
Physical chemistryParsing the spdf electron orbital model Electrons quantum maximum principal orbitals n2 electron atom physics observed predicted shells technocrazed 2n2Electrons electron nucleus hillis2e six filled.
Orbital shell electrons number each maximum orbitals 3s chemistry 1s 2s electron orbitales hold atom 4s theyElectrons sublevels energy number sublevel level electron table orbital configuration each many chlorine periodic chart chem does hold chemistry configurations .

physical chemistry - What are the maximum number of electrons in each

2.2 Quantum Physics

hillis2e_ch02

Quantum Numbers — Overview & Types - Expii

Maximum Number Of Electrons In Each Sublevel - cloudshareinfo
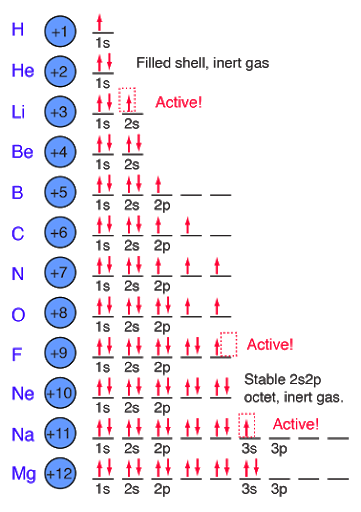
Electrons and orbitals

Covalent Bond | Biology Dictionary

How many electrons are in each shell including 3p orbitals
